基础脚本更新--Seurat(V5) + 排污 + 去双细胞传参脚本
原创基础脚本更新--Seurat(V5) + 排污 + 去双细胞传参脚本
原创
追风少年i
修改于 2026-01-06 11:13:42
修改于 2026-01-06 11:13:42
作者,Evil Genius
这会儿到过年的这段时间,基本都是更新脚本。
也不知道为什么,可能有国内拆分试剂等不清楚的原因,单细胞数据的细胞数量虽然有了很多大的提升,但是数据质量却有所下降,导致排污和去除双细胞成了分析的必须。
今天我们就来更新一下这个内容,确保单细胞数据的准确性以及后续单细胞空间联合的可靠性。
还是封装传参类的脚本,首先准备文件,共5列内容,样本名称,数据路径,数据格式(10x or csv),样本分组、疾病分期,当然,大家最好把更多的信息放进来,比如性别,年龄等内容,信息越全越好。
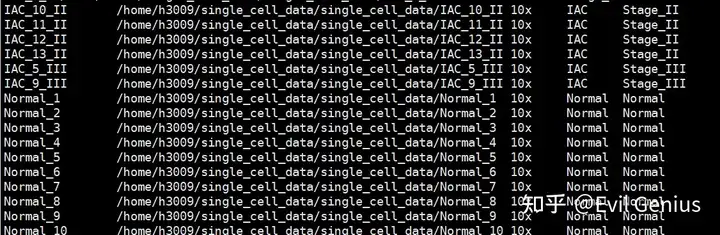
然后是脚本设计,通常来讲,我们Seurat V5 + decontX + scDblFinder的组合,每个样本单独运行decontX + scDblFinder,然后进行harmony整合分析。
全流程脚本如下,推荐大家学一学封装,这样以后传参分析数据即可,不用一行一行的运行,更多的时间用在课题设计,生物学解读,阅读文献等方面。
脚本基本都测试过了,完全满足分析需求。
#!/usr/bin/env Rscript
####zhaoyunfei
####20260104
suppressPackageStartupMessages({
library(Seurat)
library(dplyr)
library(harmony)
library(future)
library(celda)
library(Matrix)
library(ggplot2)
library(patchwork)
library(argparse)
library(Matrix)
library(tibble)
library(SingleCellExperiment)
library(scater)
library(data.table)
library(scDblFinder)
})
# ============================================================================
# 命令行参数解析
# ============================================================================
parser <- ArgumentParser(description = "单细胞RNA-seq分析管道 - Seurat V5 Harmony整合")
# 必需参数
parser$add_argument("--sample-file", type = "character", required = TRUE,
help = "样本信息文件路径 (制表符分隔)")
parser$add_argument("--output-dir", type = "character", default = "./results",
help = "输出目录")
# 输出参数
parser$add_argument("--output-prefix", type = "character", default = "scRNA",
help = "输出文件前缀")
# QC参数
parser$add_argument("--min-cells", type = "integer", default = 3,
help = "每个基因最少表达的细胞数")
parser$add_argument("--min-features", type = "integer", default = 200,
help = "每个细胞最少检测到的基因数")
parser$add_argument("--max-features", type = "integer", default = 6000,
help = "每个细胞最多检测到的基因数")
parser$add_argument("--max-mito", type = "double", default = 10,
help = "线粒体基因百分比阈值")
parser$add_argument("--min-counts", type = "integer", default = 500,
help = "每个细胞最少UMI数")
# 分析参数
parser$add_argument("--nfeatures", type = "integer", default = 2000,
help = "用于可变基因分析的特征数")
parser$add_argument("--npcs", type = "integer", default = 30,
help = "PCA主成分数")
parser$add_argument("--dims", type = "integer", default = 20,
help = "用于降维的PC数")
parser$add_argument("--resolution", type = "double", default = 0.5,
help = "聚类分辨率")
# Harmony参数
parser$add_argument("--harmony-vars", type = "character", default = "sample_id",
help = "Harmony整合的变量")
# 运行参数
parser$add_argument("--threads", type = "integer", default = 8,
help = "并行线程数")
parser$add_argument("--seed", type = "integer", default = 42,
help = "随机种子")
args <- parser$parse_args()
# ============================================================================
# 设置环境
# ============================================================================
cat("Seurat V5 Harmony整合管道\n")
cat("参数设置:\n")
for (arg_name in names(args)) {
cat(sprintf(" %-20s: %s\n", arg_name, args[[arg_name]]))
}
cat("\n")
# 创建输出目录
if (!dir.exists(args$output_dir)) {
dir.create(args$output_dir, recursive = TRUE)
}
# 设置并行计算
if (args$threads > 1) {
plan("multicore", workers = args$threads)
options(future.globals.maxSize = 30 * 1024^3)
}
set.seed(args$seed)
# ============================================================================
# 1. 读取样本信息
# ============================================================================
cat("步骤1: 读取样本信息\n")
sample_info <- read.delim(args$sample_file, stringsAsFactors = FALSE)
colnames(sample_info) <- c("sample_name", "sample_path", "data_format", "group", "stage")
cat("找到", nrow(sample_info), "个样本:\n")
print(sample_info)
# ============================================================================
# 2. 读取和处理单个样本
# ============================================================================
cat("步骤2: 读取样本数据\n")
read_single_sample <- function(sample_name, sample_path, data_format, group, stage) {
cat(sprintf("读取样本: %s\n", sample_name))
if (data_format == "10x") {
# 读取10x数据
data <- Read10X(data.dir = sample_path)
counts <- as.matrix(data,'dgCMatrix')
sce <- SingleCellExperiment(assays = list(counts = counts))
qc_stats <- perCellQCMetrics(sce)
keep_cells <- qc_stats$sum >= 500 & qc_stats$detected >= 200
sce_filtered <- sce[, keep_cells]
sce_filtered <- computeLibraryFactors(sce_filtered)
sf <- sizeFactors(sce_filtered)
if (any(sf <= 0)) {
warning(sprintf("发现 %d 个细胞的size factors <= 0,正在修复...",
sum(sf <= 0)))
sf[sf <= 0] <- min(sf[sf > 0])
sizeFactors(sce_filtered) <- sf
}
decontX_res <- decontX(sce_filtered)
data = assays(decontX_res)$decontXcounts
obj <- CreateSeuratObject(
counts = data,
project = sample_name,
min.cells = args$min_cells,
min.features = args$min_features
)
} else if (data_format == "csv") {
# 读取CSV数据
expr_matrix <- read.csv(sample_path, row.names = 1,check.names = F)
counts <- as(as.matrix(expr_matrix), "dgCMatrix")
sce <- SingleCellExperiment(assays = list(counts = counts))
qc_stats <- perCellQCMetrics(sce)
keep_cells <- qc_stats$sum >= 500 & qc_stats$detected >= 200
sce_filtered <- sce[, keep_cells]
sce_filtered <- computeLibraryFactors(sce_filtered)
sf <- sizeFactors(sce_filtered)
if (any(sf <= 0)) {
warning(sprintf("发现 %d 个细胞的size factors <= 0,正在修复...",
sum(sf <= 0)))
sf[sf <= 0] <- min(sf[sf > 0])
sizeFactors(sce_filtered) <- sf
}
decontX_res <- decontX(sce_filtered)
data = assays(decontX_res)$decontXcounts
obj <- CreateSeuratObject(
counts = expr_matrix,
project = sample_name,
min.cells = args$min_cells,
min.features = args$min_features
)
} else {
stop(sprintf("不支持的数据格式: %s", data_format))
}
# 添加元数据
obj$sample_id <- sample_name
obj$orig.ident <- sample_name
obj$group <- group
obj$stage <- stage
# 计算QC指标
obj[["percent.mt"]] <- PercentageFeatureSet(obj, pattern = "^MT-|^mt-")
obj[["percent.ribo"]] <- PercentageFeatureSet(obj, pattern = "^RP[SL]|^Rp[sl]")
# 重命名细胞
obj <- RenameCells(obj, add.cell.id = sample_name)
cat(sprintf(" 细胞数: %d, 基因数: %d\n", ncol(obj), nrow(obj)))
return(obj)
}
# 读取所有样本
seurat_list <- list()
for (i in 1:nrow(sample_info)) {
row <- sample_info[i, ]
obj <- read_single_sample(row$sample_name, row$sample_path,
row$data_format, row$group, row$stage)
seurat_list[[row$sample_name]] <- obj
}
# ============================================================================
# 3. 创建Seurat V5对象
# ============================================================================
cat("步骤3: 创建Seurat V5对象\n")
for (i in 1:length(seurat_list)){
obj = seurat_list[[i]]
sce <- as.SingleCellExperiment(seurat_list[[i]])
sce <- scDblFinder(sce)
obj$scDblFinder_score <- sce$scDblFinder.score
obj$scDblFinder_class <- sce$scDblFinder.class
obj_clean <- subset(obj, subset = scDblFinder_class == "singlet")
seurat_list[[i]] = obj_clean
}
seurat_merged <- seurat_list[[1]]
if (length(seurat_list) > 1) {
for (i in 2:length(seurat_list)) {
sample_name <- names(seurat_list)[i]
cat(sprintf("合并样本 %d/%d: %s\n", i, length(seurat_list), sample_name))
seurat_merged <- merge(
x = seurat_merged,
y = seurat_list[[i]]
)
}
}
# 添加统一的元数据
metadata_df <- do.call(rbind, lapply(names(seurat_list), function(sample_name) {
obj <- seurat_list[[sample_name]]
# 直接从元数据中获取(如果存在)
if ("group" %in% colnames(obj@meta.data) && "stage" %in% colnames(obj@meta.data)) {
data.frame(
row.names = colnames(obj),
sample_id = sample_name,
group = obj$group,
stage = obj$stage,
stringsAsFactors = FALSE
)
} else {
# 如果元数据中没有,使用默认值
n_cells <- ncol(obj)
data.frame(
row.names = colnames(obj),
sample_id = rep(sample_name, n_cells),
group = rep("Unknown", n_cells),
stage = rep("Unknown", n_cells),
stringsAsFactors = FALSE
)
}
}))
seurat_merged <- AddMetaData(seurat_merged, metadata = metadata_df)
cat("合并后总细胞数:", ncol(seurat_merged), "\n")
cat("合并后基因数:", nrow(seurat_merged), "\n")
# ============================================================================
# 4. 质量控制
# ============================================================================
cat("步骤4: 质量控制\n")
# 重新计算QC指标(为了确保一致)
seurat_merged[["percent.mt"]] <- PercentageFeatureSet(seurat_merged, pattern = "^MT-|^mt-")
original_cells <- ncol(seurat_merged)
# 过滤细胞
seurat_filtered <- subset(seurat_merged,
subset = nFeature_RNA > args$min_features &
nFeature_RNA < args$max_features &
nCount_RNA > args$min_counts &
percent.mt < args$max_mito)
cat("过滤前:", original_cells, "个细胞\n")
cat("过滤后:", ncol(seurat_filtered), "个细胞\n")
cat("过滤掉:", original_cells - ncol(seurat_filtered), "个细胞\n")
# QC可视化
qc_plots <- VlnPlot(seurat_filtered,
features = c("nFeature_RNA", "nCount_RNA", "percent.mt"),
group.by = "sample_id",
pt.size = 0.1,
ncol = 3)
ggsave(file.path(args$output_dir, paste0(args$output_prefix, "_QC_violin.pdf")),
qc_plots, width = 15, height = 5)
# ============================================================================
# 5. 数据标准化和预处理
# ============================================================================
cat("步骤5: 数据标准化和预处理\n")
# 标准化
seurat_filtered <- NormalizeData(seurat_filtered)
# 寻找高变基因
seurat_filtered <- FindVariableFeatures(seurat_filtered,
selection.method = "vst",
nfeatures = args$nfeatures)
# 缩放数据
seurat_filtered <- ScaleData(seurat_filtered)
cat("找到", length(VariableFeatures(seurat_filtered)), "个高变基因\n")
# 高变基因可视化
var_plot <- VariableFeaturePlot(seurat_filtered)
top10 <- head(VariableFeatures(seurat_filtered), 10)
var_plot <- LabelPoints(plot = var_plot, points = top10, repel = TRUE)
ggsave(file.path(args$output_dir, paste0(args$output_prefix, "_variable_genes.pdf")),
var_plot, width = 10, height = 8)
# ============================================================================
# 6. PCA降维
# ============================================================================
cat("步骤6: PCA降维\n")
# 运行PCA
seurat_filtered <- RunPCA(seurat_filtered,
features = VariableFeatures(object = seurat_filtered))
# 肘部图
elbow_plot <- ElbowPlot(seurat_filtered, ndims = 50)
ggsave(file.path(args$output_dir, paste0(args$output_prefix, "_elbow_plot.pdf")),
elbow_plot, width = 8, height = 6)
cat("PCA计算完成\n")
# ============================================================================
# 7. Seurat V5 Harmony整合
# ============================================================================
cat("步骤7: Seurat V5 Harmony整合\n")
cat("使用 IntegrateLayers 进行 Harmony 整合...\n")
# 关键步骤:为每个样本设置layer
seurat_filtered$orig.ident <- seurat_filtered$sample_id
# 执行Harmony整合
seurat_harmony <- IntegrateLayers(
object = seurat_filtered,
method = HarmonyIntegration,
orig.reduction = "pca",
new.reduction = "harmony",
verbose = FALSE,
assay = "RNA"
)
cat("Harmony整合完成\n")
# 检查整合结果
cat("可用降维方法:", names(seurat_harmony@reductions), "\n")
# ============================================================================
# 8. UMAP降维
# ============================================================================
cat("步骤8: UMAP降维\n")
# 基于Harmony整合后的空间运行UMAP
seurat_harmony <- RunUMAP(seurat_harmony,
reduction = "harmony",
dims = 1:min(args$dims, ncol(seurat_harmony[["harmony"]])),
reduction.name = "umap.harmony",
reduction.key = "UMAPHARMONY_")
# 可视化
umap_by_sample <- DimPlot(seurat_harmony,
reduction = "umap.harmony",
group.by = "sample_id",
pt.size = 0.5,
label = FALSE)
umap_by_group <- DimPlot(seurat_harmony,
reduction = "umap.harmony",
group.by = "group",
pt.size = 0.5,
label = FALSE)
umap_by_stage <- DimPlot(seurat_harmony,
reduction = "umap.harmony",
group.by = "stage",
pt.size = 0.5,
label = FALSE)
# 组合图
combined_umap <- umap_by_sample + umap_by_group + umap_by_stage
ggsave(file.path(args$output_dir, paste0(args$output_prefix, "_UMAP_harmony.pdf")),
combined_umap, width = 15, height = 5)
# 按样本分面显示
faceted_umap <- DimPlot(seurat_harmony,
reduction = "umap.harmony",
group.by = "sample_id",
split.by = "sample_id",
ncol = min(4, length(unique(seurat_harmony$sample_id))),
pt.size = 0.5)
ggsave(file.path(args$output_dir, paste0(args$output_prefix, "_UMAP_by_sample.pdf")),
faceted_umap,
width = min(15, 4 * length(unique(seurat_harmony$sample_id))),
height = 10)
# ============================================================================
# 9. 细胞聚类
# ============================================================================
cat("步骤9: 细胞聚类\n")
# 基于Harmony整合后的空间构建邻居图
seurat_harmony <- FindNeighbors(seurat_harmony,
reduction = "harmony",
dims = 1:min(args$dims, ncol(seurat_harmony[["harmony"]])))
# 聚类分析
seurat_harmony <- FindClusters(seurat_harmony, resolution = args$resolution)
# 聚类可视化
cluster_plot <- DimPlot(seurat_harmony,
reduction = "umap.harmony",
group.by = "seurat_clusters",
label = TRUE,
pt.size = 0.5) +
ggtitle(paste("Clusters (resolution =", args$resolution, ")"))
ggsave(file.path(args$output_dir, paste0(args$output_prefix, "_clusters.pdf")),
cluster_plot, width = 10, height = 8)
cat("找到", length(unique(seurat_harmony$seurat_clusters)), "个cluster\n")
# 聚类统计
cluster_stats <- as.data.frame(table(seurat_harmony$seurat_clusters))
colnames(cluster_stats) <- c("Cluster", "Cell_Count")
cluster_stats$Percentage <- round(cluster_stats$Cell_Count / sum(cluster_stats$Cell_Count) * 100, 2)
write.csv(cluster_stats,
file.path(args$output_dir, paste0(args$output_prefix, "_cluster_stats.csv")),
row.names = FALSE)
cat("聚类统计:\n")
print(cluster_stats)
# ============================================================================
# 10. 差异表达分析
# ============================================================================
cat("步骤10: 差异表达分析\n")
seurat_harmony = JoinLayers(seurat_harmony)
# 设置默认ident为cluster
Idents(seurat_harmony) <- "seurat_clusters"
# 寻找所有cluster的marker基因
cat("寻找marker基因...\n")
all_markers <- FindAllMarkers(seurat_harmony,
only.pos = TRUE,
min.pct = 0.25,
logfc.threshold = 0.25,
test.use = "wilcox")
# 保存所有marker基因
write.csv(all_markers,
file.path(args$output_dir, paste0(args$output_prefix, "_all_markers.csv")),
row.names = FALSE)
cat("找到", nrow(all_markers), "个marker基因\n")
# 提取每个cluster的前10个marker基因
top10_markers <- all_markers %>%
group_by(cluster) %>%
top_n(n = 10, wt = avg_log2FC)
write.csv(top10_markers,
file.path(args$output_dir, paste0(args$output_prefix, "_top10_markers.csv")),
row.names = FALSE)
# 热图可视化(显示每个cluster的前5个marker基因)
top5_markers <- all_markers %>%
group_by(cluster) %>%
top_n(n = 5, wt = avg_log2FC)
# 创建热图
if (nrow(top5_markers) > 0) {
heatmap_genes <- unique(top5_markers$gene)
if (length(heatmap_genes) > 0) {
heatmap_plot <- DoHeatmap(seurat_harmony,
features = heatmap_genes,
group.by = "seurat_clusters",
size = 3) +
theme(axis.text.y = element_text(size = 6))
ggsave(file.path(args$output_dir, paste0(args$output_prefix, "_marker_heatmap.pdf")),
heatmap_plot,
width = 12,
height = max(8, length(heatmap_genes) * 0.15))
}
}
# ============================================================================
# 11. 保存结果
# ============================================================================
cat("步骤11: 保存结果\n")
# 保存RDS文件
rds_file <- file.path(args$output_dir, paste0(args$output_prefix, "_harmony_integrated.rds"))
saveRDS(seurat_harmony, file = rds_file)
cat("保存RDS文件:", rds_file, "\n")
# 保存元数据
metadata <- seurat_harmony@meta.data
write.csv(metadata,
file.path(args$output_dir, paste0(args$output_prefix, "_metadata.csv")),
row.names = TRUE)
# 保存整合信息
integration_summary <- data.frame(
Parameter = c("Samples", "Cells_Before_QC", "Cells_After_QC",
"Genes", "Clusters", "Resolution", "Integration_Method"),
Value = c(nrow(sample_info),
original_cells,
ncol(seurat_harmony),
nrow(seurat_harmony),
length(unique(seurat_harmony$seurat_clusters)),
args$resolution,
"Harmony (IntegrateLayers)")
)
write.csv(integration_summary,
file.path(args$output_dir, paste0(args$output_prefix, "_integration_summary.csv")),
row.names = FALSE)生活很好,有你更好。
原创声明:本文系作者授权腾讯云开发者社区发表,未经许可,不得转载。
如有侵权,请联系 cloudcommunity@tencent.com 删除。
原创声明:本文系作者授权腾讯云开发者社区发表,未经许可,不得转载。
如有侵权,请联系 cloudcommunity@tencent.com 删除。
评论
登录后参与评论
推荐阅读
目录

